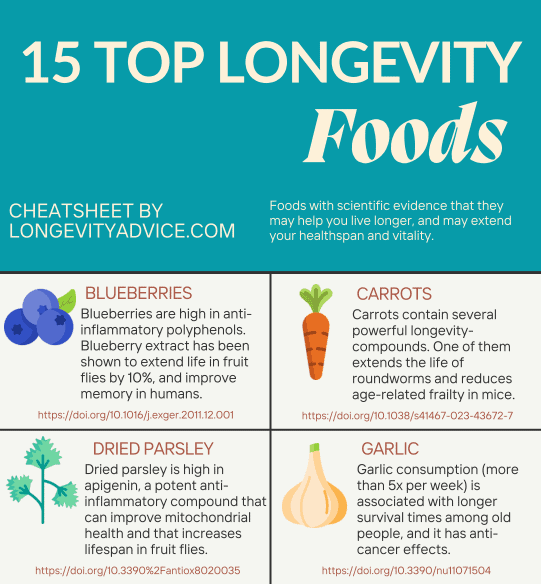Is microbiome longevity a key component of overall longevity?
Will splurging on an expensive probiotic like Seed, or drinking Kombucha every day really make a difference in your lifespan?
While the exact answer isn’t exactly yes or no, research on microbiome longevity interventions do suggest that there are things you can do to improve the health and longevity of your gut microbiome.

Among others, these methods range from the Mediterranean diet, to probiotic supplementation, and fecal microbiota transplant (FMT).
But before we get into the details, you may be wondering:
What is the gut microbiome, and what does research say about its relationship with aging?
Table of Contents
The community that lives inside you
The microbiome is the diverse community of microorganisms, including bacteria, viruses, fungi, and other microbes, that inhabit various environments in and on your body.
The gut microbiome specifically refers to the microorganisms that reside in your gastrointestinal (GI) tract, including your large intestine, small intestine, and stomach.
To describe this microbial community’s influence, combine the two sayings:
“You are what you eat” — Your diet largely determines the types of microbes that live in your gut. Eating whole, fiber-rich plant foods and fermented foods will help proliferate the “good” bacteria in your gut, while sugary, processed foods will feed the “bad” ones.
Microbe populations compete for dominance in your gut. Your dietary choices largely dictate which ones thrive.
“You are the average of the five people you spend the most time with” — More likely than not, you share many of the same microbe strains with the people you live with or date! And in early life, you acquire most microbes from your mother.
These microbes inside you have cravings, which may impact your cravings. Some studies show correlation between microbes and mental health, including how you respond to stress.
Besides mental health, microbes play critical roles in metabolism, immunity, and digestion. With all of these functions in mind, I naturally wondered how the gut microbiome is involved in human longevity.
How does your gut microbiome composition impact the aging process?
Or the reverse: Does aging itself change your gut microbiome?
Research shows that the relationship works both ways: As you get older, your microbiome composition changes with both age itself, and age-related diseases. However, in a reciprocal manner, the microbiome also impacts the way your body ages.
Functions of your gut microbiome
The gut microbiome aids various bodily processes, including:
- Digestion: Microbes help break down complex carbs and fiber, producing short-chain fatty acids (SCFAs) that are crucial for gut health!
- Immunity: Microbes communicate with immune cells to fight infection.
- Vitamin synthesis: Microbes produce enzymes that synthesize vitamins B1, B9, B12, and K.
- Nutrient metabolism: Microbes metabolize nutrients, including amino acids, vitamins, and lipids.
- Drug metabolism: Microbes encode enzymes that can activate drugs, deactivate them, or convert them into other metabolites.
- Protection from pathogens
- Bile metabolism: Microbes break down bile from your liver, allowing bile acids to be reabsorbed and reused.
- Gut-brain-axis: Certain bacteria stimulate production of neurotransmitters like serotonin, glutamate, and gamma-aminobutyric acid (GABA).
What makes up a ‘healthy’ microbiome?
Your relationship with most of your microbes is symbiotic, or mutually beneficial. While microbes provide the functions mentioned above, your body provides them with food and shelter!

There is no universal definition of what a ‘healthy’ microbiome is. That said, while scientists have not defined exactly what type or amount of certain microbes is required, one review in Gut states:
“A healthy gut… is not only ‘free from diagnosed diseases’ but also ‘operates without causing any discomfort or signs of dysfunction.”
For your gut to be free of disease and dysfunction, a ‘well-balanced’ and diverse gut microbiota is one crucial factor.
Signs of an unhealthy gut may include an upset stomach, bloating, unexpected weight changes, fatigue, skin irritation, autoimmune conditions, or food intolerances.
An unbalanced gut microbiome is often referred to as “dysbiosis.”
There are three types of dysbiosis, which are caused by:
- Loss of good gut bacteria
- Overgrowth of harmful bacteria
- Loss of gut microbe diversity (both good and bad bacteria)
These types of imbalance are not mutually exclusive. In fact, they often occur at the same time.
According to Microorganisms author, Tomas Hrncir, the microbial changes that cause dysbiosis can result from:
- Genetic predispositions
- Infections or inflammation
- Diet (high sugar, low fiber)
- Xenobiotics (drugs, antibiotics, food additives)
- Hygiene
Dysbiosis can lead to “disruption of the gut barrier, and imbalance of the host immune and metabolic systems.”
The gut microbiome helps maintain your gut barrier, which protects your intestines from toxins and pathogens, while allowing for nutrient absorption.
As we age, our gut becomes more susceptible to dysfunction and permeability, aka “leaky gut.” (By permeability, I mean literal gaps in your intestinal lining that enlarge.)
Gaps in your gut lining allow more inflammatory signaling molecules and bacteria to travel into your bloodstream, making leaky gut a major source of inflammation.
As humans, roundworms, fruit flies, fish, mice, and monkeys age, intestinal barrier dysfunction becomes more common.
Do age-related microbiome changes play a role in this increase in leaky gut incidence with age?
Researchers conducting randomized control studies (RCTs) wondered the same thing. And it turns out that in multiple species, microbe changes can contribute to leaky gut… keep reading to find out how!
Microbes impact longevity in multiple species
Randomized controlled studies (RCTs) on model organisms like mice and Drosophila (fruit flies) are crucial to understand the relationship between microbes and aging, without interference from endless variables that impact our microbiomes in day-to-day life.
Researchers today still grapple with the fundamental question: Does a “healthy” microbiome promote healthy aging, or is a balanced microbiome a product of certain age-related changes and lifestyle?
RCTs play an important role in answering this question, as explained in a 2024 review:
“Another important knowledge gap is to understand whether changes in gut microbiota profile drive unhealthy or healthy ageing and longevity or, in contrast, age-related changes in host physiological functions and lifestyle patterns (particularly diet) determine gut microbiota composition and metabolic function during ageing. Although this knowledge gap can be narrowed by performing large and long-term longitudinal human trials, studies using a simple model organism such as the fruit fly, Drosophila melanogaster, demonstrated that changes in microbiota composition may precede aging.”
One study, conducted on Drosophila, found that sharp changes in flies’ microbes (dysbiosis) predicted future loss of intestinal barrier function (aka “leaky gut”)—a major predictor of mortality. After leaky gut occurred, the flies experienced even more dysbiosis, causing significant immune system activation, and death.
To reinforce their findings, the researchers created a group of axenic (microbe-less) flies. These flies lived longer because they experienced dysbiosis and leaky gut later in life!

I found this information important because it shows how microbiome dysbiosis can lead to physiological changes in flies’ intestines that ultimately impact their lifespan.
Similar outcomes were seen in mice: microbe-free mice lived longer (due to delayed leaky gut), and microbe changes predicted leaky gut– which then predicted mortality.
This doesn’t mean that a complete lack of microbes is always beneficial. However, it does show that in these species, preventing age-onset dysbiosis can aid life extension!
Interestingly, this study also suggested that individual variability in microbiota can predict individual variability in lifespan. While flies with normal microbiomes lived a wide range of lifespans (between 20-80 days), microbe-less flies almost all died at approximately day 80.
I also found these interesting tidbits from RCTs on the microbiome and overall longevity, such as:
- Feeding young flies microbes from older flies decreased their lifespan and increased incidence of intestinal barrier dysfunction.
- Feeding older flies microbes from younger donors extended their lifespan.
- Transferring microbes from younger to older African killifish “ameliorated behavioral decline and extended lifespan.”
- Germ-free mice who received fecal matter from an obese mouse developed a different body composition than those who received fecal matter from the obese mouse’s non-obese twin.
As you can see, these RCTs demonstrate how microbes can impact lifespan in a variety of species. With this background knowledge, let’s dive into what the human studies report!
How your gut microbiome changes with age
While changes are highly variable due to environmental and personal factors, there are a few key ways in which your gut microbiome may change with age:
- Diversity changes
According to a 2023 review, older individuals often show “a general decrease in microorganism diversity and probiotics, together with an increase in opportunistic agents that could be related to age-related chronic diseases.”
- Shift in bacterial composition
Certain bacterial species, such as Akkermansia, are consistently more abundant with aging. Others, like Faecalibacterium, Bacteroidaceae, and Lachnospiraceae, generally reduce with aging. Similarly, a 2022 review indicated an age-related loss of “dominant commensal taxa (group of organisms),” which are then replaced by a second group of organisms.
- Functional changes
Older adults tend to have less pathways involved in carbohydrate metabolism and amino acid synthesis. However, some oldest-old adults had more short chain fatty-acid production compared to young-old adults.
- Changes as a result of disease
Conditions such as gastrointestinal disorders, Type 1 diabetes, liver disease, rheumatoid arthritis, and obesity are associated with changes in microbiome composition.
According to researchers conducting the (iHMP), loss or gain of certain microbes may also be associated with “the state of preterm birth, IBD and prediabetes in individuals.” This project’s team hopes to eventually identify specific gut microbiome states that function as predictive biomarkers of certain diseases.
Human gut microbiome aging interventions
The main microbiome-based therapeutic interventions include:
- Probiotics: live microorganisms (usually bacteria or yeast)
- Prebiotics: carbohydrates found in high-fiber foods that the body can’t digest, but microbes can!
- Synbiotics: probiotics with a prebiotic
- Postbiotics: bioactive compounds produced when good gut bacteria digest and break down prebiotics.
- Dietary regimens: eating certain types of foods such as whole or fermented foods, or adhering to regimens like the Mediterranean diet.
One last intervention you may have heard of is fecal microbiota transplant (FMT), the transferring of a healthy donor’s bacteria to a recipient’s colon.
FMT was shown to extend the lifespans of mice and killifish. However, a 2023 meta-analysis noted that while FMT has been carried out successfully in humans to prevent C. diff. infection, “clinical research on FMT with regard to human aging has yet to be carried out, despite the gut microbiota being explored in various pathologies or diseases, such as frailty, diabetes, cancer, cardiovascular disease, and Alzheimer’s disease.”
While it’s difficult to directly pinpoint microbiome changes that impact aging, restoring a healthy microbiome state can help prevent disease and discomfort. According to Nature,
“The aim of such interventions is to break the self-perpetuating cycle of ageing-linked physiological decline facilitated by a disease-susceptible microbiome.”
While your response to each type of intervention is highly personal, and research for certain methods is limited by small sample size, short experimental duration, or monitoring of host physiology/well-being only (no microbiome profiling), research has suggested positive results from each of these treatments.
So what microbiome longevity intervention best suits your goals for life extension?
How to enhance your microbiome longevity for healthy aging
Microbiome-focused dietary changes are one of the most common interventions demonstrated to positively impact overall health and wellbeing.
That said, since your gut microbiome is highly individualized, there is no solid consensus of a specific, fool-proof intervention that will improve your microbiome and extend your lifespan.

Yet even if you try one of the following suggestions and your microbiome remains unchanged, you will still enjoy the other longevity benefits of a healthy, whole-foods focused diet!
If your microbiome is unbalanced or unhealthy, your individual response may vary based on your stage of microbiome deterioration.
For example, if populations of key, diet-responsive, beneficial microbes in your gut have completely depleted, “simple dietary interventions are likely to fail or have limited efficacy… such scenarios will require combinatorial therapy involving diet adjustment supplemented by microbial restoration of keystone taxa at the central nodes of microbiome networks.”
If you’re interested in a detailed analysis of your microbiome, it may be worth checking out some gut health tracking companies out there!
For a healthier, more balanced microbiome, research suggests you should aim to consume:
Diverse diet
A diet rich in fruits, vegetables, whole grains, and fermented foods is associated with greater microbial diversity, which is correlated with lower chronic disease risk. In fact, in countries with the highest life expectancies, “a diet rich in micronutrients and low in saturated fats… is a common denominator.”
Certain dietary prebiotic components, such as , (GOS), and also “exert anti-aging effects.”
Mediterranean diet
The Mediterranean diet is another dietary regimen you may want to try! While the diet’s exact components vary from region to region, the plant-based diet emphasizes healthy fats, fruits, vegetables, and whole grains.
One study found that adherence to the Mediterranean diet (MD) increased overall microbiome metabolic activity (measured by an increase in SCFAs). In addition, this study and numerous others associate plant-based diets like the MD with increases in beneficial bacteria, such as Bifidobacterium and Lactobacillus spp, and reduction of the pathogenic Bacteroides and Clostridium versions. In addition, increases in Oscillospira and Butyricimonas, which are linked to “health and leanness” were observed.
Probiotic-rich foods
Probiotics are live bacteria and yeasts that are good for you! Eating probiotic-rich foods has been shown to reduce inflammation and enhance immune function, which may contribute to longevity.
Fermented foods are probiotic-rich foods that are produced by the growth and metabolic activity of live microbe cultures. As a result of this process, many fermented foods like yogurt are rich in live microbes.
According to a Stanford School of Medicine Study, a diet rich in fermented foods, such as yogurt, kefir, kimchi, and fermented vegetables, resulted in a greater overall increase in microbial diversity and immunity than a high fiber diet alone. While fiber is crucial to maintain gut microbiome diversity, the study suggested that consistent consumption of fermented foods may be more beneficial to increase diversity, reduce inflammation, and decrease “risk of diabetes, cancer, and cardiovascular disease.
There is no exact recommended daily intake for probiotic-rich foods. In 2021, a Stanford study observed improved microbiome diversity, reduced inflammation, and less activation of immune cells in individuals who consumed a diet “rich” in fermented foods— defined as six servings daily.
Yet this amount is not necessarily healthy for everyone, especially if your gut is already imbalanced.

Prebiotic-rich foods
These indigestible fibers (which are complex carbs!) help feed and proliferate the “good” bacteria in your gut. They’ve been linked to bowel movement regulation, immune system enhancement, improved bone density, and reduction of risk of cardiovascular disease, type 2 diabetes, and colorectal cancers.
Prebiotics are naturally found in fruits, legumes, whole grains, and vegetables. For example, some high prebiotic foods include psyllium husk, leeks, garlic, green bananas, wheat, onions, asparagus, dandelion greens, and chicory root.
Probiotic supplements
In terms of probiotic supplements, according to a 2023 review, “the efficacy of probiotics also relies on the specificity of the strains, the dosage, the duration of the treatment, as well as on individual differences.”
According to the NIH Office of Dietary Supplements, the seven most common categories of microbes utilized in probiotic products are Lactobacillus, Bifidobacterium, Saccharomyces, Streptococcus, Enterococcus, Escherichia, and Bacillus.
Within these broad categories (genus) of microbes, probiotics can be categorized by species. Within species, there are various bacterial strains. (Genus > Species > Strain)
For example: Lactobacillus acidophilus CL1285
- Lactobacillus is the genus
- Acidophilus is the species
- CL1285 is the strain
Different strains of the same species may target different parts of your body, or exhibit different effects. If you’re taking probiotics for a specific condition, make sure to confirm that the strain name listed on your supplement exactly matches the one you’re looking for!

Probiotic supplement dosages are based on the amount of bacterial colony forming units (CFUs). Most pills range from 1 to 10 billion CFUs, and research shows that in order to reap the benefits, 100 million to 1 billion microbes must make it to your intestine.
These supplements can expire, and often must be refrigerated. The microbes in your probiotic pill capsules, powders, or liquids may die as they reach their expiration date, so purchasing a supplement with at least 1 billion CFUs is important.
While some manufacturers recommend taking probiotic supplements with food, others suggest taking them on an empty stomach.
Lactobacillus and Bifidobacterium seem to survive best when taken on an empty stomach, whereas Saccharomyes boulardii can be taken with or without food. Since lactobacillus microorganisms rely on glucose for survival in acidic conditions, research shows they may survive better if taken with carbs or sugar.
Finally, I suggest you make sure your probiotics are third-party-tested. This ensures they contain the bacterial species and dosages they say do, and aren’t contaminated.
Lifestyle habits
Apart from dietary changes, studies show your microbiome will benefit if you:
- Exercise regularly: Improves circulation to your gut, and aids stronger gut contractions for bowel movements.
- Get adequate sleep: One study found healthy microbiomes to also promote better sleep!
- Stay hydrated: Drinking water has been associated with higher microbial diversity.
Things to avoid for microbiome longevity
If your goal is to acquire a more balanced, healthy microbiome, it is crucial to reduce your intake of sugar, artificial sweeteners, fatty foods, and highly processed foods.
Your gut microbiome is very sensitive to your food choices; diets that are low-fiber, high-fat, and high-protein lead to reduced gut microbiota diversity “as quickly as one day.”
Acute alcohol consumption can also disrupt your microbiome. Drinking is linked to reduced SCFA production and depletion of both good and bad bacteria in the gut; however, one study showed that synbiotic treatment after drinking can help restore some depleted beneficial bacteria species. (The 24-strain probiotic mix administered to participants was the Seed Health SH-DS01 capsule).
Finally, a quick note on antibiotics. These medications kill bacteria, or prevent their growth to prevent infections. While some antibiotics specifically target harmful bacterial species better than others, most broad-spectrum antibiotics can kill both good and bad bacteria in your body.
For this reason, antibiotics can harm microbial diversity. If you need to take them, taking a probiotic with Lactobacillus rhamnosus GG and/or Saccharomyces boulardii during and after antibiotic use can reduce risk of C. diff. overgrowth, and antibiotic-associated diarrhea.
Can probiotic supplements work without a healthy diet?
With all of this information in mind, it seems that your microbes benefit from the same healthy habits (eating whole foods, staying hydrated, exercising, eating fiber, etc.) that you do!
And while it would be great to try to incorporate as many of these recommendations as possible into our daily lives, I wondered if consuming probiotic supplements alone, without many overall diet changes, would make noticeable improvements in my gut health. (I try to avoid processed foods and sugar, but I admit I don’t always hit the mark in terms of fiber and vegetable consumption…I’d describe my current diet as more Western than Mediterranean 🙁 )
I quickly realized that my question is a quite common topic of debate on the internet.
In the sources I reviewed, it seems that if your microbiome is already healthy, supplements may help diversify your gut, but drastic changes are unlikely.
If you have a poor diet, taking a supplement alone may help improve your gut health, but in order to sustain positive microbiome changes, a healthy diet that nourishes those good microbes is necessary!
However, as I previously mentioned, if certain key bacterial species in your gut are depleted past a certain point, simple dietary changes may not restore them. In this case, the Nature article recommended a combination of dietary changes and specific probiotic supplements to restore key bacterial species and improve microbiome health.
If you think you may be experiencing dysbiosis (common symptoms include digestive issues, acne, fatigue, urinary issues, vaginal itching, etc.), it is important to visit a doctor, who may run tests to see what bacteria, fungi, or yeasts are present, and personal make health recommendations from there.
Whether you have an unbalanced microbiome or not, eating foods that foster proliferation of healthy microbes is clearly important. One helpful tip I found to remember what types of foods to eat is the “four phoenetic Fs.”
The four phonetic Fs include:
- Fiber: whole grains, nuts, beans, seeds, fruits, and vegetables
- Healthy fats: avocados, olives, and foods high in omega-3s (or other monounsaturated and polyunsaturated fatty acids)
- Phenols: colorful fruits and vegetables, such as red peppers, purple cabbage, and blueberries.
- Fermented foods: kimchi, yogurt, sauerkraut, kombucha, etc!
In terms of supplements, remember that they are not all the same. Certain strains work better for certain purposes, such as reducing inflammation or improving UV radiation protection!
While I’ve listed quite a few studies describing specific strains and their corresponding health benefits, most probiotic supplements on the market are made up of a combination of multiple beneficial strains.
I’m curious about what specific brands or combinations are most effective for my own gut health and personal longevity goals, and if you are too, stay tuned for my next piece!
Microbiome and longevity FAQs
Do probiotics work without prebiotics?
Prebiotics are indigestible complex carbohydrates that your body can’t digest, but your microbes can! They feed the “good” bacteria in your gut and are found in whole foods such as bananas, artichokes, and asparagus. While you don’t necessarily need to take prebiotics with probiotics for them to work, frequently consuming prebiotics is important to sustain a diverse, healthy microbiome.
Are probiotics good for you?
Probiotics are the beneficial live microorganisms found in fermented foods and many dietary supplements. Consuming fermented foods and probiotic supplements with the right bacterial strains can help proliferate healthy gut microbe populations that support metabolism, digestion, vitamin synthesis, immunity, and more!
How to heal your microbiome after antibiotics?
Both during and after antibiotics use, taking probiotic supplements may help maintain your gut microbiome health. Supplements that specifically contain the bacterial strains Lactobacillus rhamnosus GG and/or Saccharomyces boulardii have been shown to reduce risk of C. diff overgrowth and antibiotic-associated diarrhea.
What promotes a healthy microbiome?
Consistently eating a diet rich in fiber, whole foods, and fermented foods, and low in sugar, processed foods, and fat can promote the health of your gut microbiome. Avoiding antibiotics, exercising regularly, staying hydrated, and getting enough sleep also benefit gut health. Probiotic supplements can help diversify your microbiome and promote growth of good bacteria— just make sure they contain the right strains for you, and are third-party tested!
I joined the Longevity Advice team shortly after college, where I studied Physiology and Human Biology & Society. I’m excited to explore a range of topics through the lens of longevity, such as contraceptives, the epigenome, and biohacking. Apart from writing, I enjoy making jewelry, exercising, and nail art!